Aesthetics Products
Introducing Novo's Plasma IQ/RevivaMask Usage Program. Contact Us Today to Learn More!
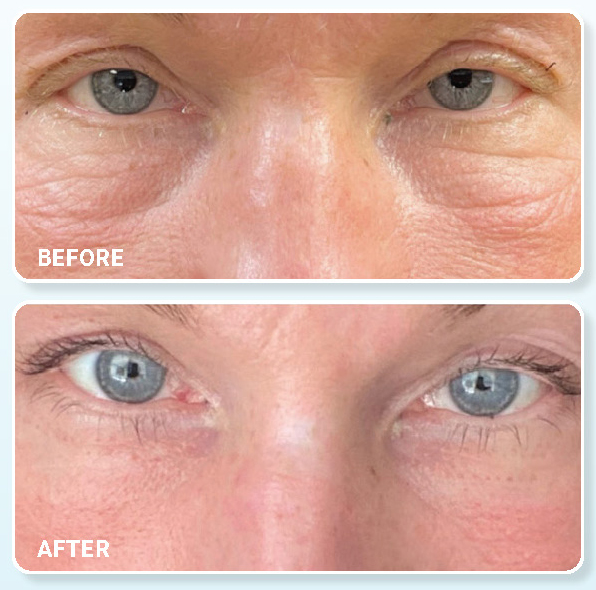
Plasma IQ Handheld Energy Device
Plasma IQ is the first ever FDA Class II 510(k) cleared hand-held plasma energy device and the only one with published histology data in human skin. Plasma IQ is a minimally invasive, ablative technology that harnesses the power of plasma energy to rejuvenate and tighten skin, and reduce the signs of aging. Two levels of energy (high 950V – low 650V) allow for safe and effective treatments for a variety of indications and areas. ₁

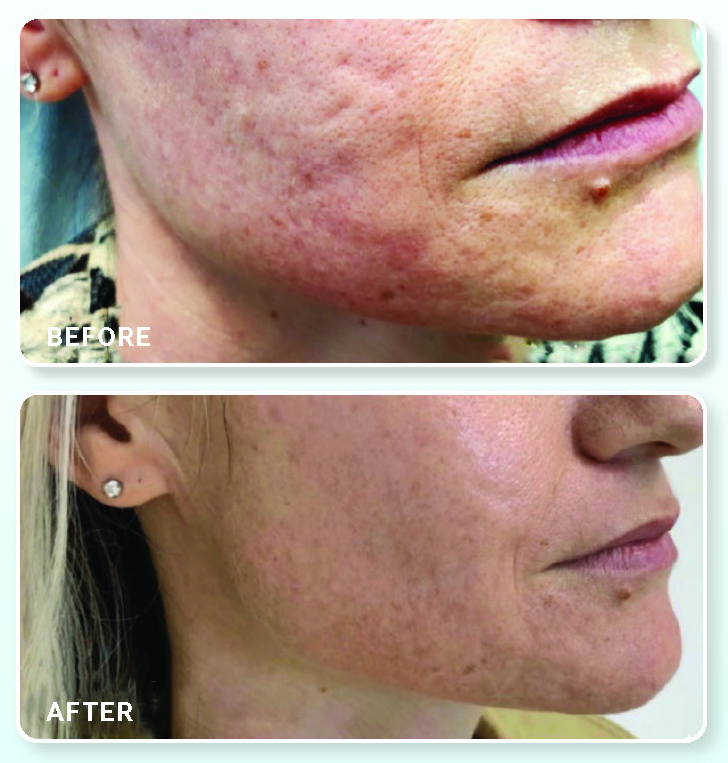

Extracellular Infused Mask
This highly-compressed mask sheet maximizes absorption of the extracellular matrix solution that consists of a powerhouse mixture of UCT-MSC (umbilical cord tissue – mesenchymal stem cell) derived growth factors, cytokines, proteins, extracellular vesicles including exosomes and interleukins. To enhance effectiveness and outcomes, the extracellular solution should be used for all post-aesthetic procedure care and will complement any practice in providing a much-needed post-aesthetic care solution. The cooling sensation of this powerful, medical-grade product provides a soothing and calming effect to post-procedure skin, which can be sensitive and unpleasant at times. It is recommended to use this product after micro-needling, derma peels, and laser treatment type applications, and can be used as a regular skincare routine for anti-aging and skin imperfections. ₁
Allogeneic Extracellular Vesicles
Amniotic fluid-derived extracellular vesicles growth factors and cytokines are known to be non-tumorigenic. They are intended to be used with an abrasive technique such as micro-needling, in both hair restoration and skin rejuvenation applications. AF EV’s are available in 2cc vials with 200 billion EV’s and must be deep frozen.
Cord blood-derived exosomes are backed by published, peer-reviewed studies on process and efficacy. These products are intended to be used with an abrasive technique such as micro-needling, and are available in a number of volumes and concentrations, and must be deep frozen. ₁


Autologous Extracellular Vesicles
Our autologous extracellular vesicle product is produced via a proprietary, plasma precipitate method containing a specific fraction of proteins, derived from whole blood. This process yields hundreds of billions of extracellular vesicles per mL, is completely natural, with no culture expansion, radiation or addition of compounds. This product is manufactured under ISO-7 cGMP standards, liquid inoculation and endotoxin testing is performed on all lots. This product is produced in accordance with FDA 21 CFR 1271.15(b) standards, same surgical procedure exemption (i.e. platelet rich plasma.) ₁
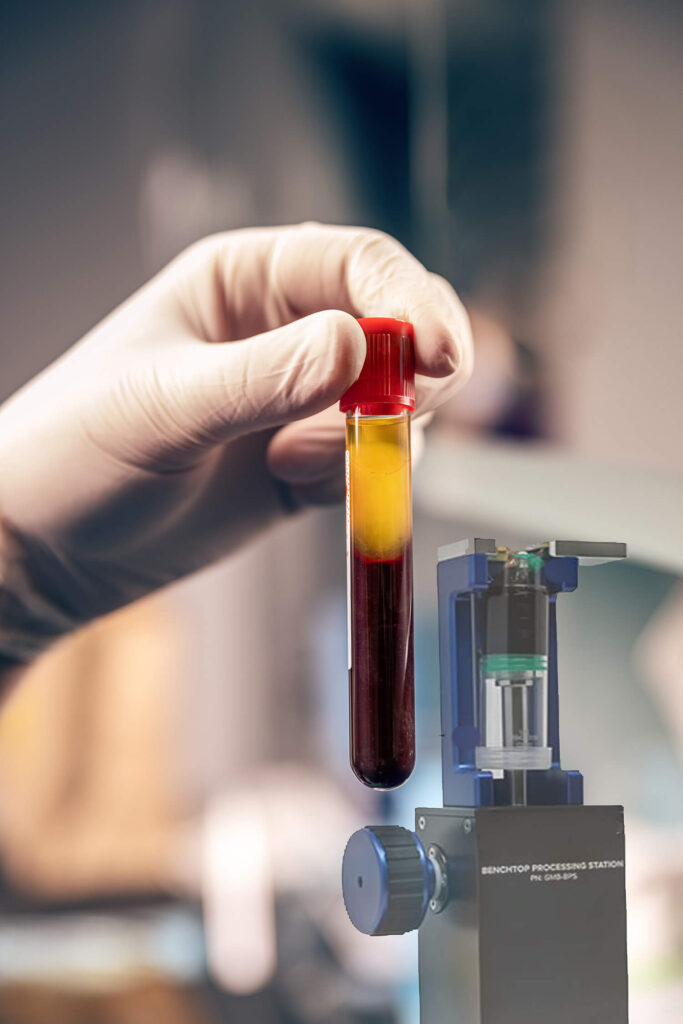
Platelet Rich Plasma / Platelet Rich Fibrin
Our platelet rich plasma system allows you to capture with confidence the entire buffy coat, consistently obtaining 7-9x times concentration, 9-20 billion total platelets, depending on the size of end sample. The system also allows for the flexibility of tailored treatments via easily choosing leukocyte rich or leukocyte poor samples. This system is FDA 510(k) approved. ₁

Amniotic Fluid Allograft
Amniotic fluid functions as a supportive cushion and a protective environment including rich sources of nutrients, cytokines and growth factors that are required for development and maturation. Our manufacturer’s proprietary process preserves the original relevant characteristics of the amniotic fluid. Amniotic fluid possesses properties that make it attractive for use in clinical applications (FGF, EGF, PDGF, VEGF, TGFbeta, HA, IL-1 RA, TIMP-1 and TIMP-2). Our amniotic fluid products are ethically collected in accordance with current good tissue practice standards (cGTP), donor and fluid are confirmed negative by FDA registered and CLIA-certified labs (HBsAg, HBcAb, HVCAb, HIVI/2 Ab, HCV NAT, HIV NAT, HBV NAT, RPR/STS, HTLVI/11, and WNV), and terminally sterilized for patient safety. The products are shelf stable and are currently in FDA IND-approved, Phase II multi-center trials (PGF201.) ₁
₁ All manufacturer materials supporting claims made on this page are available upon request.
Contact NOVO today to learn how we can enhance the health and wellness of your patients and your practice.
All rights reserved.
