Flowable Products
Autologus Products

Autologous Extracellular Vesicles
Our autologous extracellular vesicle product is produced via a proprietary, plasma precipitate method containing a specific fraction of proteins, derived from whole blood. This process yields hundreds of billions of extracellular vesicles per mL, is completely natural, with no culture expansion, radiation or addition of compounds. This product is manufactured under ISO-7 cGMP standards, liquid inoculation and endotoxin testing is performed on all lots. This product is produced in accordance with FDA 21 CFR 1271.15(b) standards, same surgical procedure exemption (i.e. platelet rich plasma.) ₁
Platelet Rich Plasma (Leukocyte Rich & Leukocyte Poor)
Our platelet rich plasma system allows you to capture with confidence the entire buffy coat, consistently obtaining 7-9x times concentration, 9-20 billion total platelets, depending on the size of end sample. The system also allows for the flexibility of tailored treatments via easily choosing leukocyte rich or leukocyte poor samples. This system is FDA 510(k) approved. ₁
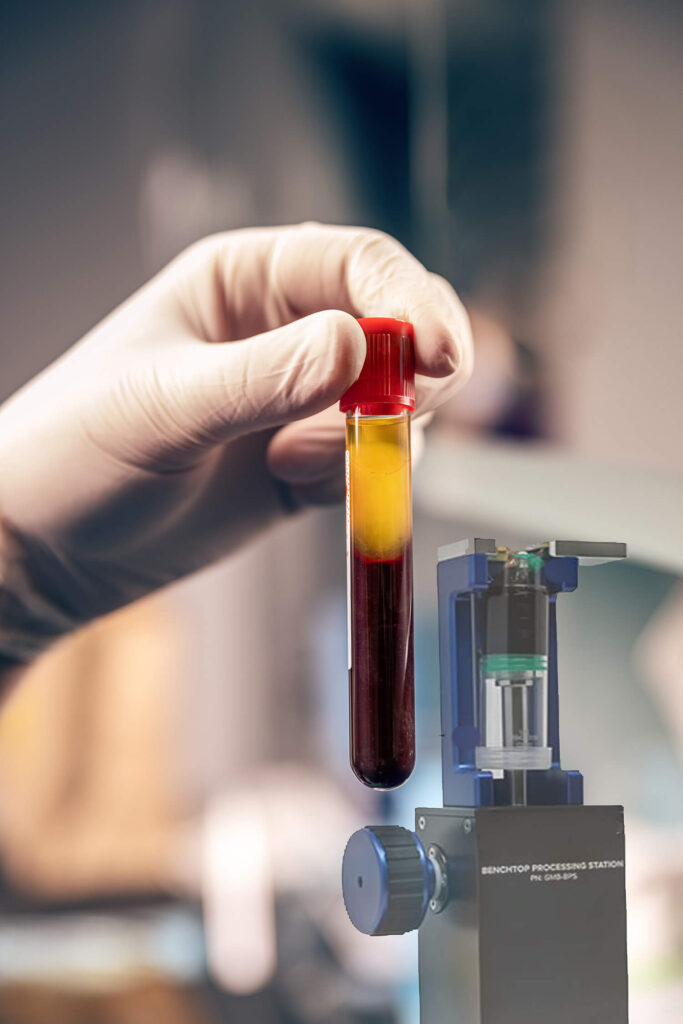
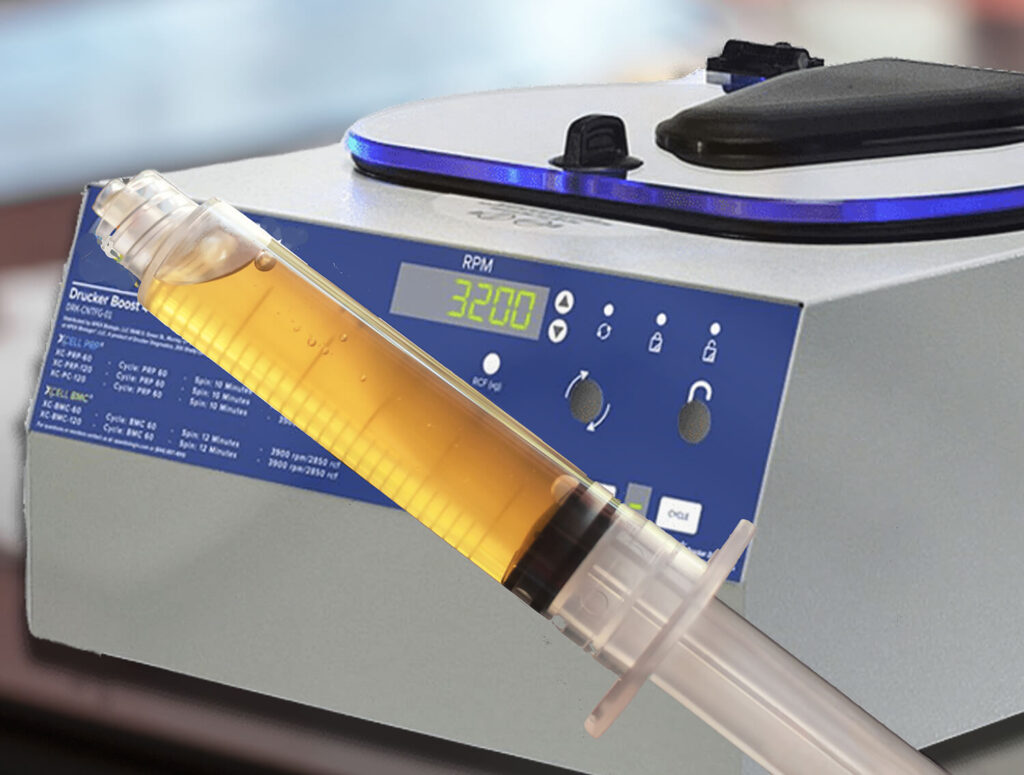
Protein Concentrate (A2M & IRAP)
Our protein concentrate system allows for the simple and easy processing of protein poor plasma into best-in-class concentrations of desired proteins like A2M and IRAP. Scientific evidence points to A2M (Alpha 2 Macroglobulin) as a potential key to slowing the progression of osteoarthritis at the molecular level. A2M is a broad spectrum, multi-purpose protease inhibitor that potentially captures and inactivates three major chemicals known to contribute to joint breakdown and cartilage damage. Research on IRAP (Interleukin-1 Receptor Antagonist Protein) has shown that it specifically prevents IL-1 from binding to its receptors, potentially slowing the progression of osteoarthritis. This system is FDA 510(k) approved. ₁
Bone Marrow Concentrate (MSC's)
Our bone marrow concentrate system is revolutionary in its ability to maximize MSC count in such an affordable and easy to use system. This system has a specialized device that helps shorten the processing time to just 12 minutes and achieve best in class concentration levels. The system captures the buffy coat with up to 85% of total nucleated cells, a 5.5x times baseline over BMA, and up to 7cc of MSC’s. This system is FDA 510(k) approved. ₁

₁ All manufacturer materials supporting claims made on this page are available upon request.
Frequently asked Questions
Novo offers cutting edge regenerative medicine solutions, community and referrng clinician education programs, and practice administrative solutions ranging from patient consultation aids, patient financing options, and support to keep your practice adherent to the most current legal and regulatory environment of regenerative medicine.
Unlike most distributors that ongoingly change products they represent based on their financial benefit, Novo has a more long-term view on supporting our practices. Our close relationships with leading manufacturers, clinical leaders, top regenerative medicine legal counsel, highly experienced coding and billing experts, etc. We are committed to conducting business in a manner that builds more durable and reliable relationships with our practices.
Achilles’ tendinosis, ALS, Alzheimer’s disease, cancer, corneal disease / cataracts / glaucoma, disc degeneration in spine, heart disease, leukemia, liver disease, lymphoma, lupus, meniscus tears, osteoarthritis (shoulder, hip, knee, wrist), plantar fasciitis, Parkinson’s disease, rotator cuff injuries, spinal cord injuries, stroke, tendinitis (elbow, shoulder, knee, wrist), type I diabetes, wound (injury, burns, pad, DVT).
- Univ. of Miami: https://umiamihealth.org/en/treatments-and-services/sports-medicine-institute
- Univ. of Nebraska: https://www.unmc.edu/stemcells/educational-resources/faqs.html
- National Library of Medicine: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6492284/
In ancient times, Chinese, South American, and Egyptian civilizations all used vegetable, mineral and skin grafting to treat wounds. In the early 1800s, scientists revealed all life depends on chemical reactions within cells and could be reproduced in the lab. In the early 1900s, scientists discovered DNA and its impact on cell division and subsequently were able to reproduce this phenomena in labs. In the mid 1900s organ transplantation, surgical implants and bio-materials led to the ability to grow and harvest tissues for therapeutic use, hence the birth of the field we now call regenerative medicine.
Some regenerative medicine therapies are covered by medicare and private insurances, but in many instances they are not. Considering the average single patient deductible in the U.S. Is $4490 and the average coinsurance is 75%, in many instances regenerative medicine therapies are less expensive than traditional therapies. With Novo’s strategic partners in patient financing, many of these therapies can be less expensive than a patient’s monthly cable and internet bill.
- eHealthInsurance.com. “ACA Index Report on Unsubsidized Consumers in the 2021 Open Enrollment Period.”
- https://www.debt.org/medical/health-insurance-premiums; NOV 2020
- https://www.healthcare.com/coinsurance-15648; MARCH 2022
Contact NOVO today to learn how we can enhance the health and wellness of your patients and your practice.
All rights reserved.
